Symbol Glossary
ISO 15223-1 Explanation of the symbols used on Diamatrix packaging

MANUFACTURER
ISO: 15223-1:2016
Medical devices — Indicated the medical device manufacturer, as defined in EU Directives 90/385/EEC, 93/42/EEC and 98/79/EC. This symbol is accompanied by the name and address of the manufacturer. (Ref No. 5.1.1)

AUTHORIZED REPRESENTATIVE IN THE EUROPEAN COMMUNITY
ISO: 15223-1:2016
Medical devices — Indicates the authorized representative in the European Community. This symbol is accompanied by the name and address of the authorized representative in the European Community. (Ref No. 5.1.2)
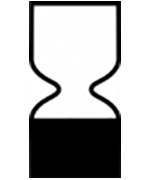
USE-BY DATE
ISO: 15223-1:2016
Medical devices — Indicates the date after which the medical device is not to be used. Date format: YYYY-MM-DD. (Ref No. 5.1.4)

BATCH CODE
ISO: 15223-1:2016
Medical devices — Indicates the manufacturer’s batch code so that the batch or lot can be identified (Ref No. 5.1.5)

CATALOG NUMBER
ISO: 15223-1:2016
Medical devices — Indicates the manufacturer’s catalog number so that the medical device can be identified (Ref No. 5.1.6)

SERIAL NUMBER
ISO: 15223-1:2016
Medical devices — Indicates the manufacturer’s serial number so that a specific medical device can be identified (Ref No. 5.1.7)

STERILIZED USING IRRADIATION
ISO: 15223-1:2016
Medical devices — Indicates a medical device that has been sterilized using irradiation (Ref No. 5.2.4)
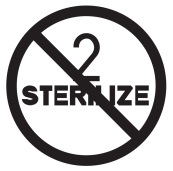
DO NOT RESTERILIZE
ISO: 15223-1:2016
Medical devices — Indicates a medical device that is not to be resterilized (Ref No. 5.2.6)
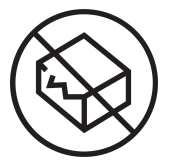
DO NOT USE IF PACKAGE IS DAMAGED
ISO: 15223-1:2016
Medical devices — Indicates a medical device that should not be used if the package has been damaged or opened. (Ref No. 5.2.8)
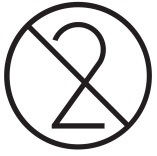
DO NOT RE-USE/ SINGLE USE/ USE ONLY ONCE
ISO: 15223-1:2016
Medical devices — Indicates a medical device that is intended for one use, or for use on a single patient during a single procedure. (Ref No. 5.4.2)

CONSULT INSTRUCTIONS FOR USE
ISO: 15223-1:2016
Medical devices — Indicated the need for the user to consult the instructions for use and where the electronic instructions for use (eIFU) and symbols glossary can be found. (Ref No. 5.4.3)

EUROPEAN CONFORMITY
ISO: 15223-1:2016
Medical devices — European conformity (CE) mark for Class I medical devices. (Ref 2007/47/EC)

EUROPEAN CONFORMITY
ISO: 15223-1:2016
Medical devices — European conformity (CE) mark with Notified Body identification number for Class IIa, IIb, III medical devices. Notified Body No. 1639: SGS Belgium NV (Ref 2007/47/EC and Regulation (EU) 2017/745)
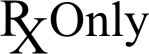
PRESCRIPTION ONLY
ISO: 15223-1:2016
Medical devices — CAUTION: U.S. Federal law restrict this device to sale, distribution and use by or on the order of a licensed physician. (Ref No. 21 CFR 801.109)